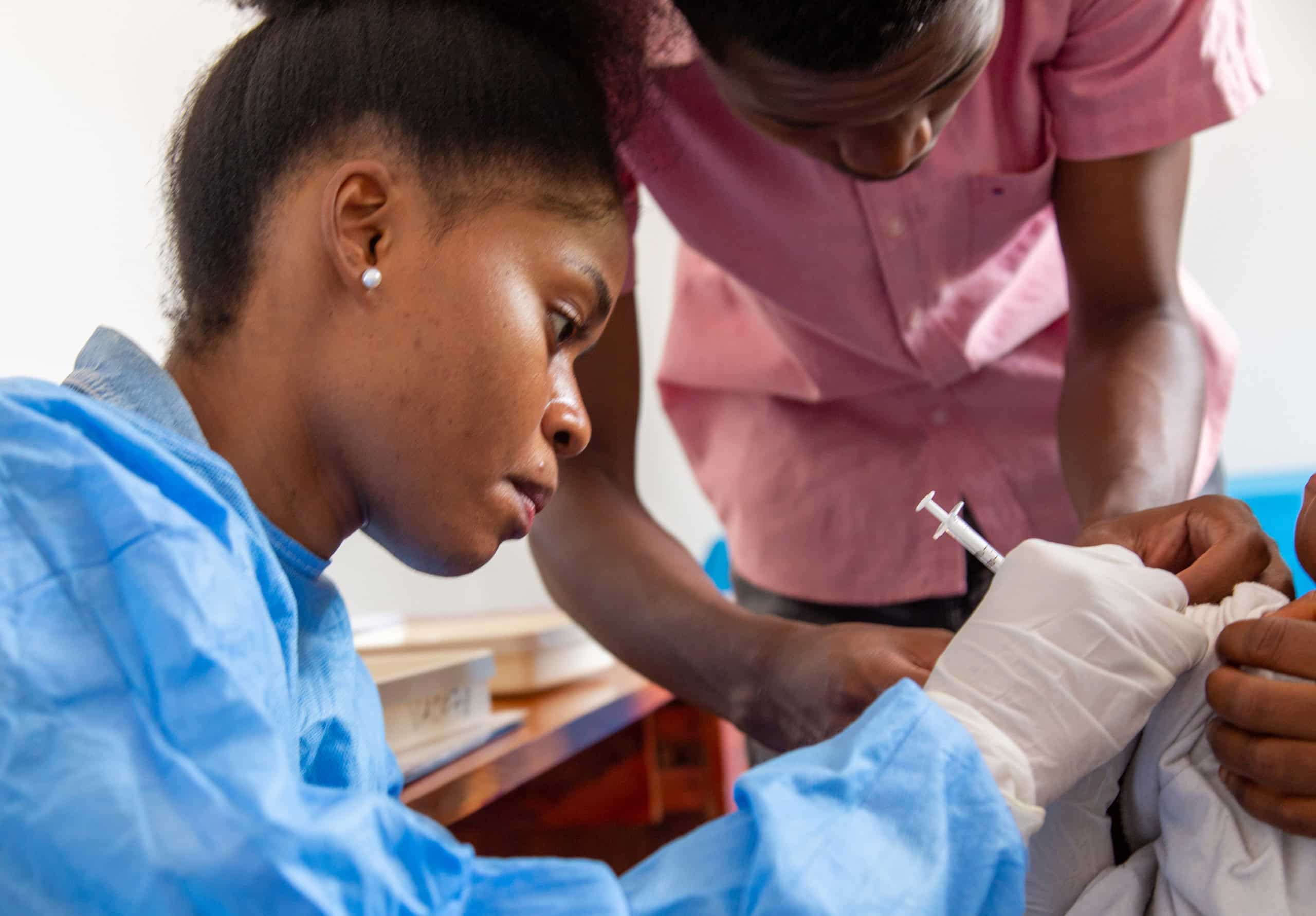
The UNICEF has secured a four-year supply of the newly WHO-approved R21/Matrix-M vaccine that could revolutionize malaria prevention, especially in Africa where 90% of fatalities occur.
Malaria, one of the most widely-known parasitic diseases to mankind, takes the life of a child every minute according to World Health Organization (WHO) estimates, with almost 620,000 deaths per year. A staggering 241 million people contract the disease annually, illustrating the global scope of this public health crisis.
Now, a new vaccine has joined the world in the fight against the disease, having obtained WHO approval earlier this month. The United Nations Children’s Fund (UNICEF) has entered into an agreement to procure a four-year supply of the newly approved R21/Matrix-M vaccine from mid-2024.
The new vaccine is a product of over 35 years of vaccine research, a collaboration between the University of Oxford and the Serum Institute of India, notable in that it is only the second malaria vaccine approved for public use. The first, known as RTS,S, initiated the ground-breaking work in this field but was less efficacious.
Dubbed R21/Matrix-M, it is the first to surpass a 75% efficacy rate, providing protection of up to two years against symptomatic malaria with a single booster – which could be a ground-breaking shift in the fight to eradicate the disease, particularly on the African continent, where some 90% of the fatalities from the illness occur.
Loading...
“It is heartbreaking and unacceptable that almost half a million children die of malaria every year. This agreement is a critical step towards protecting more children from this deadly disease,” said Director of UNICEF Supply Division Leila Pakkala in a press statement announcing the signing of UNICEF’s supply agreement.
The obtaining of the WHO’s endorsement earlier this month was critical to allow international bodies such as UNICEF to begin procuring the vaccines, with WHO having vetted the safety profile and efficacy of R21/Matrix-M.
Adar Poonawalla, CEO of the Serum Institute of India, commemorated the WHO endorsement in a press release: “For far too long, malaria has threatened the lives of billions of people across the globe, disproportionately affecting the most vulnerable amongst us. This is why the WHO recommendation and approval of the R21/Matrix-MTM vaccine marks a huge milestone on our journey to combat this life-threatening disease.”
The Serum Institute of India has already begun to prepare the production capability to deliver up to 100 million doses per year, to be doubled in the coming two years.
Cost and Delivery
Critically, for a vaccine that aims to prevent severe disease primarily in lower-income countries, the R21/Matrix-M is expected to cost only between $2 and $4 per dose, with an initial regimen consisting of one dose every month for three months, and one booster within the next 12 months – meaning that a full treatment protocol offering high levels of protection for almost two years costs less than $16.
Countries have already begun to factor in the new vaccine into their epidemiological planning, with both Ghana and Nigeria having obtained approval for the vaccine’s use – Ghana too has begun establishing a vaccine factory in Accra for this purpose in collaboration with the Serum Institute of India.
The Path Forward
Despite the leaps in vaccine development, challenges persist. While vaccines like R21/Matrix-M are revolutionary, they are not the final answer to a disease as complex and historically persistent as malaria. In the interim, nations continue to deploy traditional methods like insecticides, improved housing, and swamp drainage, especially in regions where malaria remains prevalent. It’s also worth mentioning that mRNA technology, catalyzed by COVID-19 research, holds the promise of even more effective vaccines in the future.
Global efforts have already made significant strides; the WHO reports a 90% decline in malaria mortality rates in the 20th century, but with many in the world still suffering under high rates of the disease, the new vaccine could be key in reducing fatalities in the years to come.
Loading...